
Electro-Com Celebrates 21 years!
Electro-Com is Australia’s leading specialist distributor of Radio Frequency Identification (RFID) hardware and Sensor technologies, with the technical knowledge and understanding to support the wide range of products we deliver.
Our purpose is to facilitate the distribution and effective utilisation of RFID equipment across the Australasian region. Our goal is to ensure that our Integrator, Installer and Reseller customers have the right products and technical advice to deliver the best solutions for improving the operational performance of their customers.
We operate across diverse markets, including Access Control, Security, Asset Management, Vehicle Identification, Logistics/Supply Chain, Animal/Livestock Identification, Library Systems Automation, Industrial Automation and Healthcare.
Australian owned and operated, we are wholesale distributors and technical specialists, supporting integrators, installers, resellers and OEMs.
Our services
Electro-Com specialises in the distribution of RFID hardware including RFID tags, RFID readers, antennas and accessories, and provides associated technical support services. We represent a large number of leading international technology providers. Established in Australia in 2000, Electro-Com has an extended RFID industry pedigree of over 30 years, making us internationally one of the most experienced RFID technology providers.
We hold substantial local inventory to support customers in the Australian/New Zealand region. If we don’t already stock it, we pride ourselves on our ability to rapidly source the most appropriate products through our international network, at competitive prices.
We can supply all of your RFID equipment
We can specify and supply the appropriate RFID technology, following a thorough review of your project requirements. Our extensive experience and technical expertise significantly reduces your level of risk and gives you a decisive edge over the competition. We deliver reliable, high quality products at competitive prices. Guaranteed.

ATD100 UHF Desktop Reader

ATS100 Bluetooth Reader

AT907 Android PDA

Suprema Product Range

ACTAtek Combination Unit

Spotto

Net2 Plus 1 door controller, plastic enclosure, 12V PSU
Net2 Plus 1 door controller

Net2 Software Pro

Net2 Software Lite

Net2 Exit Button E75

Net2 Desktop Reader

Net2 Proximity Metal Keypad Mifare KP75

Net2 Proximity Mifare Reader P50

Net2 Proximity Keypad KP75

Net2 Proximity Keypad KP50

Net2 Proximity Reader P200

Net2 Proximity Reader P75

Net2 Proximity Reader P50

Net2 Proximity Reader P38

Net2 I/O Board

Scanndy UHF

ECCO+UHF

HyWEAR UHF

Scanndy

ECCO+

Scanndy

ECCO Lite

HyWEAR
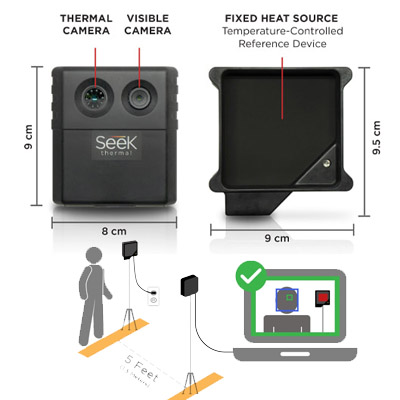
Seek Scan
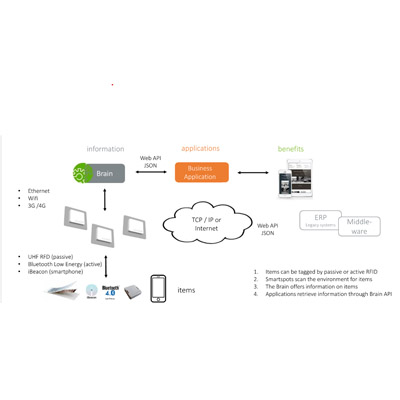
Intellifi Brain

Smartspot BLE
Smartspot Focus

Smartspot Tile

Smartspot

UHF Handheld Reader, Windows OS, IP65

UHF Ultra Rugged Antenna

UHF Ultra Slim Desktop Antenna

UHF High Performance Antenna

UHF High Gain Antenna

UHF High Performance Antenna

UHF High Performance Antenna

UHF Desktop RAIN RFID Reader
UHF Desktop RAIN RFID Reader

UHF Fixed Reader, IP54

Compact UHF Fixed Reader

All-in-One UHF Fixed Reader, integrated antenna, IP65

UHF Handheld Reader, Android OS, IP65

UHF Handheld Reader, IP65

Ultra-Compact UHF Handheld Reader, IP65

Ultra-Compact UHF Handheld Reader

UHF RFID Sled Reader

Bullnose Key Tag

Compact Key Tag

Bullnose Key Tag

ISO Card pkt 25

ISO Card

Adhesive Tag

Desktop Antenna
Micro Reader

eTag Card Reader

HF Reader Switchplate

HF Read/Write

HF Read/Write

Smart Card Reader

Wedge Reader

Wedge Reader

Desktop Reader

Desktop Reader

Desktop Reader

Wall Reader

Wall Reader

Wall Reader

Wall Reader

Wall Reader

DESFire Reader

Wall Reader

Wall Reader

Mifare Card Reader

UHF RFID Reader

UHF RFID Reader

UHF RFID Reader

UHF RFID Beam Reader

Handheld Barcode Scanner

UHF RFID Reader

Handheld UHF RFID Reader

Handheld UHF RFID Reader

Handheld UHF RFID Reader

Handheld UHF RFID Reader

Handheld UHF RFID Reader

ReadWrite 32mm Glass Transponder

ReadOnly 32mm Glass Transponder

ReadWrite 23mm Glass Transponder

ReadOnly 23mm Glass Transponder

ReadWrite Plastic Wedge Transponder

ReadOnly Plastic Wedge Transponder

ReadWrite Card Transponder

ReadOnly Card Transponder
Remote Tuning Annunciator

Remote Tuning Sender

LF Gate Antenna 200x200mm

LF Stick Antenna

Interface Board

Autotuner – Capacitance bank

Antenna Tuning Board, LF

RF Module

RF Module

LF Reader Control Board

Door Controller

Mifare Read/Write

Mifare Desktop Sector Reader

Mifare Sector Reader

Mifare Sector Reader

Mifare Desktop Reader

Mifare Desktop Reader

Mifare Reader

RFID Desktop Reader

RFID Desktop Reader

RFID Reader

RFID Reader

RFID Reader

Panel Antenna

Panel Antenna

Antenna
Antenna

Ethernet Module

Wired Module
Bluetooth Module

IP66 Housing

Long Range Reader

Mid-Range LF Reader

Evaluation Kit

Ferrite Rod

Ferrite Rod

Adaptor

Reader Module

Stick Reader

Stick Reader

Integrated LF Reader

Handheld LF Reader

Handheld LF Reader
LF Module
LF Module
LF Reader

Transponder Interface

Digital Signature Transponder

Disk Transponder

24mm Circular Inlay

32mm Glass Transponder

23mm Glass Transponder

23mm Glass Transponder

12mm Glass Transponder

32mm Glass Transponder

23mm Glass Transponder

12mm Glass Transponder

Vehicle Access Control System

Long Range Reader

Long Range Antenna

Long Range Antenna

Antenna

Antenna

Antenna
Antenna

Antenna

Reader Module

Reader Module

Handheld Reader

Handheld Reader

Mid Range Reader

Mid Range Reader Module

Directional Antenna

Gate Antenna

Gate Antenna

Compact Long Range Reader

Compact Long Range Reader

Fixed Long Range Reader

Fixed Long Range Reader

Fixed Long Range Reader
Multiplexer Module
Reader Module
Reader Module

Reader Module
Reader Module

Reader Module

Card Reader

ProximityReader

Proximity Reader

Multitag Reader

Proximity Reader

Proximity Reader

Shielded Pad Reader

Proximity Reader

Desktop Reader
Mid Range Reader Module

Mid Range Reader

Handheld Reader

Handheld Reader

Handheld Inventory Reader

Crystal Gate – Excell

Crystal Gate – Excell

Crystal Gate – Wave

Crystal Gate – Wave

Base Antenna
Antenna

Handheld Antenna

Shielded Pad Antenna

Pad Antenna
Long Range Reader
Long Range Reader Module

Long Range Reader Module

Long Range Reader

Shielded Pad Antenna

UHF Compact Reader

Hybrid Barcode and RFID Wearable
Most popular products
Here is a small range of our most commonly specified products.




























